Maximum Safety, Targeted Simplicity – And Lower Costs
The next generation of Innosept Asbofill machines is setting new standards in aseptic filling
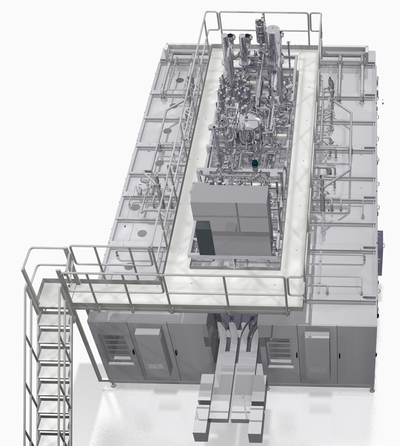
Thomas Niehr* Paul-Uwe Winterhoff** In our modern day and age the beverage industry, and the dairy and fruit juice sectors in particular, would be inconceivable without aseptic filling – not least because this filling method contains a whole host of benefits. Aseptic filling of beverages in plastic bottles, for instance, retains the natural flavor and aroma of each and every drink as well as the important vitamins these contain. Another major advantage of aseptic filling is the lack of preservatives – creating an economic advantage for the beverage industry and an additional health aspect welcomed by consumers. In the field of aseptic bottling, prior to the actual filling process more and more plants are using what is known as dry sterilization. This principle has long been in successful use in the series of classic Innosept Asbofill machines; in the next generation, namely the Innosept Asbofill ABF 611 and the Innosept Asbofill ABF 711, this has been improved even further. Compared to the machinery manufactured to date, this new family of aseptic linear fillers has many optimized features which give the user an entire spectrum of extra benefits.The dry sterilization method for various types of plastic bottle and closure The major advantage of the dry sterilization method is that all kinds of plastic bottle and closure can be reliably sterilized. In the Innosept Asbofill machine series, where dry sterilization comes into play, all surfaces are sprayed completely evenly, regardless of the size and shape of the bottles being processed. This ensures safe sterilization, even with ribbed, embossed, and square bottles and with bottles with very structured surfaces. ** Aseptic Filling Technology Manager, KHS GmbH, Bad Kreuznach, Germany. Phone: +49 (671) 852-2900 ** Design Engineering Manager, Aseptic Filling Technology, KHS GmbH, Bad Kreuznach, Germany. Phone: +49 (671) 852-2902 Hydrogen peroxide (H2O2) is used in the dry sterilization process, where H2O2 aerosol is sprayed into the plastic bottles and onto the closures. The condensation this forms on the bottle and closure surfaces is dried off with sterile hot air. The result is dry and sterile bottle and closure surfaces. In this procedure there is no need to dispose of any peracetic acid, the sterilization medium used in wet sterilization. There is also no water used, which means that no wastewater is generated and that dry sterilization saves on one of our most precious future resources. Much reduced sterile area – but still the utmost safety The size of the required sterile area where dry sterilization is applied is greatly reduced in comparison to the classical wet aseptic process. Instead of the usual 65 m3, less than 15 m3 of space is taken up by the dry sterilization unit where the rotary design principle is used. Where linear machines are deployed for dry sterilization, the size of the sterile area drops even further. This is one definitive advantage of linear technology, as seen in the next generation of Innosept Asbofill fillers. The ABF 711, for example, has a sterile area measuring just 1.5 m3; the ABF 611 requires a mere 0.9 m3 of sterile area. As only the bottle neck enters the Innosept Asbofill sterile zone, bottle exterior sterilization otherwise needed is superfluous here. The much reduced sterile area still gives the utmost aseptic safety. This safety is increased even further by the plastic bottles in the Innosept Asbofill ABF being conveyed throughout the entire machine in their own holders. No switching from one carousel to another is required, as is the case in the rotary system, thus eliminating the necessity for any mechanical intervention in the aseptic zone. All conceivable conversion and replacement measures take place in the unsterile area outside the aseptic zone. Another advantage of the Innosept Asbofill machines is that there is no need for intermediate sterilization. Fully geared towards the product to be filled, the recommended production cycle is between 48 and 72 hours. New generation of Innosept Asbofills for outputs of up to 24,000 plastic bottles/h Aseptic linear filling machines generally cover a low to medium output range, with aseptic rotary machines catering for the higher capacities. The Innosept Asbofill ABF 611 is thus capable of processing up to 12,000 plastic bottles an hour and is specifically designed for the low capacity range of 100 to 750 ml. The bottles may be up to 85 mm in diameter and up to 240 mm in height. The Innosept Asbofill ABF 711 can also fill up to 12,000 plastic bottles per hour, its standard processing sizes being bottles holding 0.25 to 2 liters with a maximum diameter of 120 mm and a maximum height of 350 mm. If required, the range of plastic bottles to be processed by the machines can also be extended to include special sizes not included in the above stipulations. Both machines are also available in the new twin version, increasing the filling volume to 24,000 bottles an hour. This twin concept combines the use of proven base technology with a reduction in the number of cost-intensive components, while boosting outputs within a minimum amount of space. Positively predestined for the dairy and fruit juice sectors Innosept Asbofill linear fillers are designed to fill still beverages into plastic bottles. Whether milk, yogurt drinks, fruit juices, or fruit juice drinks – all are doable. Pulpy beverages containing up to 20% pulp and with fiber lengths of up to 18 mm can also be processed without any trouble at all. In the future a machine variant will also become available which permits products that contain chunks to be filled, thus further expanding the area of use for this series of fillers. The Innosept Asbofill ABF 611 and the Innosept Asbofill ABF 711 are also particularly suitable for filling pharmaceuticals, provided they are liquid products for enteral feeding or health drinks, for example. Constancy of the neck ring the sole prerequisite Innosept Asbofill machines allow enormous flexibility in the design of bottle used, allowing customers to build up a broad spectrum of processible products. The only limitation is that the neck ring must remain constant; the shape of the bottle can vary as long as the maximum dimensions are not exceeded. Bottle designers are thus free to exercise their powers of imagination as they see fit, creating any number of quadratic, rectangular, or oval bottles which are all fully workable. A high level of machine efficiency is ensured even if the shape and volume of the bottles frequently changes. Reliable bottle infeed using an air conveyor The plastic bottles processed by Innosept Asbofill technology are fed into the machinery by an air conveyor. This ensures that bottles are conveyed reliably, gently, and under the best possible hygienic conditions using neck ring handling. The air conveyor is split into sections, each of which has a frequency-controlled blower that supplies the air channel and controls the conveying speed of the bottles by means of various volume flows. Innosept Asbofill machines have a standardized interface for connecting up the air conveyor, with the air conveyor continued further into the machine. The transfer point between the air conveyor and the filler is always identical and independent of variations in installation. Cyclic discharge to the cell carrier bar Bottles are fed into both the Innosept Asbofill ABF 611 and the Innosept Asbofill ABF 711 outside the aseptic zone. A chain moves past the air conveyor, at the end of which is a curve that ensures that the bottles are inserted in holders specially integrated into the chain for this purpose. From these chain holders grippers remove eight bottles per cycle on the Innosept Asbofill ABF 611 and ten bottles per cycle on the Innosept Asbofill ABF 711 and position them in a cell carrier bar. Holding the plastic bottles by their neck rings, this cell carrier bar transports them through all processing stages of the machine. The first station scans the cell carrier bar to see if each position contains a plastic bottle. Should this prove not to be the case, this is immediately registered and the vacant position excluded from the ensuing filling and capping process. Residue-free sterilization process Following this inspection the plastic bottles are transferred to the sterilization zone, where H2O2 aerosol is applied to each plastic bottle through a lance positioned in the plastic bottle and ending just below the bottle neck. This special method of aerosol spraying ensures that all areas of the plastic bottle are treated with H2O2. A two-channel system is formed in the bottle neck; while the H2O2 aerosol is sprayed into the center, the gas this displaces escapes up the sides of the lance and out of the bottle. To ensure the greatest possible safety, the aforementioned H2O2 is supplied in cycles at two stations. Two further cycles in the sterilization zone are reserved to allow the H2O2 to take effect. Within four cycles the bottles are then dried with hot air in an area separated by a partition until they are residue free. Clearly separated processes Once the plastic bottles have run through the sterilization process, they are conveyed to the filling station. There is also a partition in the machines between the sterilization and filling processes, which take place in different housings, clearly separating these aseptic zone areas from one another. This partition extends up to the cell carrier bar and only contains openings for the necks of the individual plastic bottles. Directed sterile air control Sterile air is blown permanently into the aseptic zone area and discharged downward by what's known as a positive displacement current via a perforated plate which separates off the aseptic zone. From there it enters the machine area where it is specifically extracted. The actual sterile air preparation is located in a valve manifold installed on the machine, which also provides all other media required for the production process. Blowers convey the air to the valve manifold that includes sterile candle filters. Candle filters have the advantage that they can be sterilized with steam prior to production, providing the maximum process safety. Non-contact filling process fully geared towards the product and plastic bottle type The filling process in the Innosept Asbofill machine series is carried out by two-stage, free-flow filling valves. During the filling process there is absolutely no contact between the plastic bottle and the filling valve in full accordance with the stipulations of aseptic safety. The specific configurations of the filling process are dictated by the product to be filled and the type of plastic bottle it is to be filled in. A push of a button at the operator terminal retrieves the relevant programmed settings. Volumetric filling of the plastic bottles by means of magnetic inductive flowmetering ensures exact fill levels. Overfilling is not necessary; product loss is avoided. If a changeover to a new plastic bottle size is planned, a press of a button on the operator terminal is all that's required to make the necessary changes to the filling volume. Safe cleaning process for filling valves and the aseptic area A cleaning adapter is located within the filling station which closes the filling valves for cleaning, ensuring continuous circulation during valve cleaning. The standard procedure on an Innosept Asbofill machine is to clean the filling valves in both directions, with the cleaning flow being reversed within the machine. The entire aseptic zone is always sanitized with foam and/or gel prior to production. This process ensures that each individual place inside the aseptic area is reliably reached. The cleaning process also treats materials extremely gently. Many possible, retrofittable options In both the Innosept Asbofill ABF 611 and the Innosept Asbofill ABF 711 pulp nozzles can be integrated into the filling valves if required. This option is particularly useful where fruit juices with a high pulp content are to be processed. Depending on the products to be filled, other useful options are nitrogen flushing before and flushing the head space of the plastic bottles with nitrogen after the filling process. This treatment, which further reduces the amount of oxygen in the filled bottle and thus in the beverage itself, has obvious advantages, particularly for beverages that are sensitive to oxygen, such as those containing vitamin C. Another possible option is to place a drop of liquid nitrogen in the head space of the filled plastic bottles. This method, which displaces the oxygen in the bottle head space, is especially prudent where very lightweight plastic bottles are deployed. Adding liquid nitrogen causes the pressure inside the plastic bottle to build up, thus giving the bottle greater stability and making it easier to process down the line, such as in the labeling area, during palletizing, and also when being transported. Like the other available options, such as double-filter units in place of single-filter units in the valve manifolds and coding plastic bottles during the production process, those mentioned above can also be integrated into Innosept Asbofill machines at a later stage. All this gives beverage bottlers an ideal safeguard for the future. Both seals and screw caps are possible When ordering an Innosept Asbofill ABF 611 or an Innosept Asbofill ABF 711, one decision which needs to be made is whether the bottles are to be sealed or closed with screw caps. This is an issue which varies from sector to sector of the beverage industry. Dairies, for instance, seal most of their products, whereas fruit juice bottlers are clear advocates of the screw cap. One feasible solution to the problem would to be include both closure systems in one machine; however, this has not yet been required in practice. Fully automatic sealing process The sealing process always takes place in a housing that is separate from the filling process. Consequently, the plastic bottles again pass through a partition after filling. The plastic bottle sealing process is fully automatic on both the Innosept Asbofill ABF 611 and the Innosept Asbofill ABF 711; it is therefore no longer necessary to have operators place seals in hoppers provided for this purpose. This has the advantage that aseptic safety is increased even further, while operator errors in the handling of seal plates that might result in malfunctions are prevented. A container is placed in the machine which is full of the aluminum seals to be applied. This container also acts as packaging for deliveries of seal plates. Grippers remove the seals from this container and distribute them among the individual hoppers. The seals are then taken from the hoppers by suction. The underlying side of each removed seal is sprayed with H2O2 to sterilize it and then placed in a drum with the side facing the product once the seal has been attached on the outside of the drum. The drum then undergoes a number of processing stages in coordination with the machine cycle configured for the plastic bottles. During the first cycle H2O2 aerosol is sprayed onto the seal, with another cycle reserved for the reaction time of the H2O2 aerosol. This is one cycle less than required for the sterilization of the plastic bottles, yet this reaction time is sufficient, as H2O2 aerosol can be applied to an aluminum seal at a much higher temperature than is possible during plastic bottle sterilization. This shorter reaction time means that the H2O2 on the seal dries within just two cycles. The seal is cycled further in the drum until it reaches its discharge position, where it is removed from the seal plate, and pressed and immediately sealed onto the designated plastic bottle. The major plus of immediate sealing is that the utmost aseptic safety is again ensured; the time during which the filled plastic bottle is open is deliberately kept as brief as possible. Application and closure processes for plastic caps and sports caps kept separate If plastic caps are to be applied to the bottles, these first travel from the sorter into a feed chute. Aligned in the same manner and fully coordinated with the number of plastic bottles conveyed on each cell carrier bar, the caps are then distributed across eight lanes in the Innosept Asbofill ABF 611 and across ten lanes in the Innosept Asbofill ABF 711. The caps are sterilized in exactly the same manner as the plastic bottles. H2O2 is sprayed onto the caps, allowed to react and then dry. The application of screw caps to plastic bottles has been designed down to the last detail. In a first step, a suction unit on the Innosept Asbofill machine places the plastic caps on the bottles, thus largely protecting the filled product against external influences. The capper housing is divided into two chambers, the aseptic zones of which are separated by partitions. The next stage in the process, namely screwing the plastic caps tight, is thus carried out in a separate sterile chamber. The key advantage here is that the rotary capping motion, which usually poses a greater risk in an aseptic zone, takes place in a separate sterile zone where the bottles are as good as sealed. Again, maximum aseptic safety is the motto here. Besides classic plastic screw caps, the Innosept Asbofill ABF 611 and Innosept Asbofill ABF 711 can also process sports caps. If required, this option can be retrofitted at any stage. We should mention here that if this option is added, it is possible to switch from flat cap to sports cap and back again at any time without the need for any changeover work. Any necessary alternative settings are made automatically. Closure inspection and leakage check Downstream of the capper unit an optical closure inspection is performed. After this, the bottles are clocked to a position where a gripper system removes them from the cell carrier bar and places them on the conveyor. After the aseptic filler there is a leakage check which is obligatory for an aseptic filling line. More simplicity at many levels Compared to the previous generation of machines, in the Innosept Asbofill ABF 611 and the Innosept Asbofill ABF 711 the sterilization zone has been simplified by reducing the number of components. For example, only one single servomotor is used for all drying lances, resulting in simplified maintenance and a cut in operating costs. Another improvement relates to machine accessibility, with the cable routing having been optimized even further and the H2O2 treatment unit and other devices repositioned. With regard to the measuring instruments, only components of the newest generation are earmarked for use in the Innosept Asbofill ABF 611 and Innosept Asbofill ABF 711. Twin concept available on request Yet another benefit of the new generation of Innosept Asbofill machines, i.e. the ABF 611 and the ABF 711, is that the original machine capacity can be easily doubled using the twin concept. This has the advantage that there is a clear reduction in cost compared to the investment in two individual machines, the reason being that the chief components can be shared by a twin machine. For example, if the Innosept Asbofill is twinned, only one valve manifold, one bottle conveyor downstream of the machines, one product and steam supply, one controller, one operator terminal, and one machine cladding are required. Using proven technology, this twin concept gives customers a higher machine output within a minimum amount of space while reducing investment costs. Moreover, the twin machine also allows users to keep a smaller stock of spare parts as parts are identical, again lowering company outgoings. What's more, as opposed to using two separate machines, the twin concept is much more compact and thus takes up less space. Training included In an aseptic world, each and every individual must constantly adhere to the rules. The best machine equipment is useless if essential rules of conduct are disregarded. In order to hone this awareness, on investing in Innosept Asbofill technology all those involved in line operations are specifically trained to this end. The program covers aspects from the basics of aseptic technology through care and maintenance measures to training on the operator terminal. All from one source – an important principle of aseptic operation Providing everything from one source is another important principle of aseptic operation. Interface problems are ruled out right from the start. KHS thus not only provides expert knowledge on aseptic systems in general but also throughout the entire aseptic process itself. This includes a complete beverage processing package, from sugar treatment through beverage production, product pasteurizing and sterilizing to CIP sanitizing. After all, it's important that process engineering equipment fully tailored to the process and designed entirely in compliance with aseptic criteria is always installed upstream of the actual aseptic filling technology. Precisely tailored to the demands of the industry All told, with its new generation of aseptic linear fillers, KHS offers the very system the industry demands. Maximum safety goes hand in hand with flexibility with regard to not only the product itself but also to the plastic bottles to be filled with said product and the closures used on these bottles. Fast, simple changeovers of product and bottle type satisfy a major requirement, namely to be able to fill an ever greater variety of products in small batches on the same line without loss of effectiveness and with the maximum aseptic safety. Thanks to a comprehensive package of options, which can also be integrated into Innosept Asbofill machines at a later stage, this is a machine generation that is perfectly equipped for both the present and the future.